Abstract
Introduction: Post-transplantation lymphoproliferative disorder (PTLD) is a rare disorder with few established therapies. Solid organ transplant (SOT) patients who develop PTLD are often frail. While some patients respond to reduction of immunosuppression (RI) or rituximab monotherapy, aggressive or relapsed disease frequently requires chemoimmunotherapy. Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) is a standard of care first-line chemoimmunotherapy for large B-cell lymphomas, but this regimen is often poorly tolerated by frail SOT patients with significant mortality, early discontinuation of therapy, neuropathy and cardiac toxicity. We report our institutional experience with rituximab, cyclophosphamide, etoposide, prednisone (R-CEP), as an alternative, anthracycline-sparing regimen for patients with PTLD.
Methods: We retrospectively reviewed records within the Lymphoma Program of the University of Pennsylvania to identify patients who received R-CEP for PTLD. R-CEP was administered on day 1 of each cycle, every 3 weeks for six total cycles in outpatient clinic: rituximab 375 mg/m2 IV, cyclophosphamide 750 mg/m2 IV, and etoposide 100 mg/m2 IV. Three patients received alternative etoposide dosing administered as 50mg/m2 IV on days 1-3. Prednisone was optional and administered at physician discretion; doses ranged from continuous SOT immunosuppression dose to 100 mg daily on days 1 to 5 of each cycle. Overall response rates (ORR), progression free survival (PFS), response duration (RD), and overall survival (OS) were assessed. Adverse effects were described by CTCAE v5.
Results: Thirty-four patients were identified (see Table 1 for Patient Characteristics). Eleven patients (32%) initially were managed with RI alone; 2 of 11 (18%) responded for ≥ 6 months before progressive disease (PD). Twenty-four patients (71%) received rituximab monotherapy (either initially or for PD after RI) with 14 of 24 (58%) responding for ≥ 6 months before PD. All who received RI and/or rituximab monotherapy had PD before starting R-CEP. Seven patients (21%) were first treated with R-CEP due to aggressive initial presentation of PTLD.
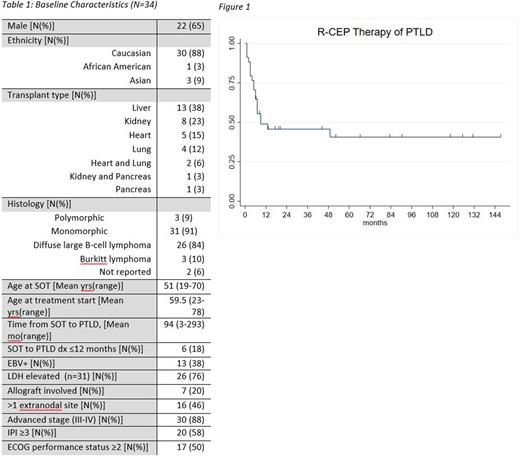
ORR at six months after completion of R-CEP was 47% with 15 complete responses (CR) and 1 partial response (PR). Median follow-up was 9.8 years (range 0.25-12 years). 46% and 41% of patients remained progression-free at 24 and 60 months, respectively (Figure 1). Median response duration (RD) was 47 months (95%CI: 5-NE). Of those who achieved CR at 6 months, median RD was not reached; 93% and 83% of patients in CR maintained responses at 24 and 60 months, respectively. Median OS was 45 months (95%CI: 13-NE), with 58% and 47% of patients alive at 24 and 60 months, respectively. Of the 21 patients who died, 12 (57%) died from PD, 2 (9%) from infections after receiving further salvage therapy, and the remainder from causes unrelated to PTLD.
Fifty percent of patients received their first cycle while hospitalized. Seventy-four percent of patients had neutropenia (41% grade 3-4 neutropenia) and 23% had febrile neutropenia. Eight patients had grade 3-4 anemia and 6 developed grade 3-4 thrombocytopenia. Ten patients (29%) required hospitalization during treatment, most commonly for febrile neutropenia, anemia, or dehydration. There were no instances of neuropathy, cardiac toxicity, or treatment-related mortality.
Of the 18 patients who did not complete all 6 cycles of chemoimmunotherapy, 11 (61%) stopped due to PD, 4 (22%) due to toxicity (most commonly cytopenias), 2 (11%) elected early stop after achievement of CR, and 1 (6%) due to a need for treatment of a secondary primary malignancy. Patients with PD commonly received R-CHOP for systemic progression (including heart transplant recipients) or radiation therapy for localized disease.
Conclusions: R-CEP is a promising alternative to R-CHOP for frail PTLD patients based on our observed efficacy and toxicity and warrants further investigation. Cytopenias were less common than historically reported for PTLD patients treated with R-CHOP (Trappe et al 2012). There was no treatment-related mortality, whereas reported treatment-related mortality with R-CHOP for PTLD is 11% (Trappe et al 2012). Study limitations include its retrospective nature, small sample size, and heterogeneous PTLD population with respect to SOT.
Disclosures
Hughes:Abbvie: Membership on an entity's Board of Directors or advisory committees; Acerta Pharma: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Genzyme: Consultancy; Janssen: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees. Dwivedy Nasta:Pharmacyclics: Research Funding; Roche: Research Funding; Rafael: Research Funding; FortySeven/Gilead: Research Funding. Svoboda:Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Atara: Consultancy; BMS: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; Pharmacyclics: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding; TG: Research Funding; ADCT: Consultancy. Landsburg:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees; Curis, Inc: Research Funding; Epizyme: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; Triphase: Research Funding. Barta:Affimed: Consultancy; Daiichi Sankyo: Consultancy; Kyowa Kirin: Consultancy, Honoraria; Seagen: Honoraria; Janssen: Other: Independent Data Monitoring Committee member; Acrotech: Honoraria. Schuster:AbbVie: Research Funding; Adaptive Biotechnologies: Research Funding; AstraZeneca: Consultancy; BeiGene: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; DTRM: Research Funding; Fate Therapeutics: Consultancy; Genentech: Consultancy, Research Funding; Genmab: Consultancy; Incyte: Consultancy, Research Funding; Juno Therapeutics: Consultancy, Research Funding; Legend Biotech: Consultancy; Loxo Oncology: Consultancy; Merck: Research Funding; MorphoSys: Consultancy; Mustang Biotech: Consultancy; Nordic Nanovector: Consultancy; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Pharmacyclics: Research Funding; Regeneron: Consultancy; Roche: Consultancy, Research Funding; TG Therapeutics: Research Funding. Chong:Novartis: Consultancy; Tessa: Consultancy; Juno/BMS: Consultancy; Beigene: Consultancy; KITE: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal